Modernizing infectious disease testing and reporting in non-traditional and under-resourced healthcare settings
Summary
COVID-19 turned places like schools and nursing homes into testing sites. We worked with the Centers for Disease Control and Prevention (CDC) to launch and scale a free, time-saving tool that makes it easy for non-traditional and under-resourced organizations to record results for diagnostic tests and quickly report critical data to public health departments. As we continue to support ongoing COVID-19 reporting, we’re expanding this tool for use with additional infectious diseases.
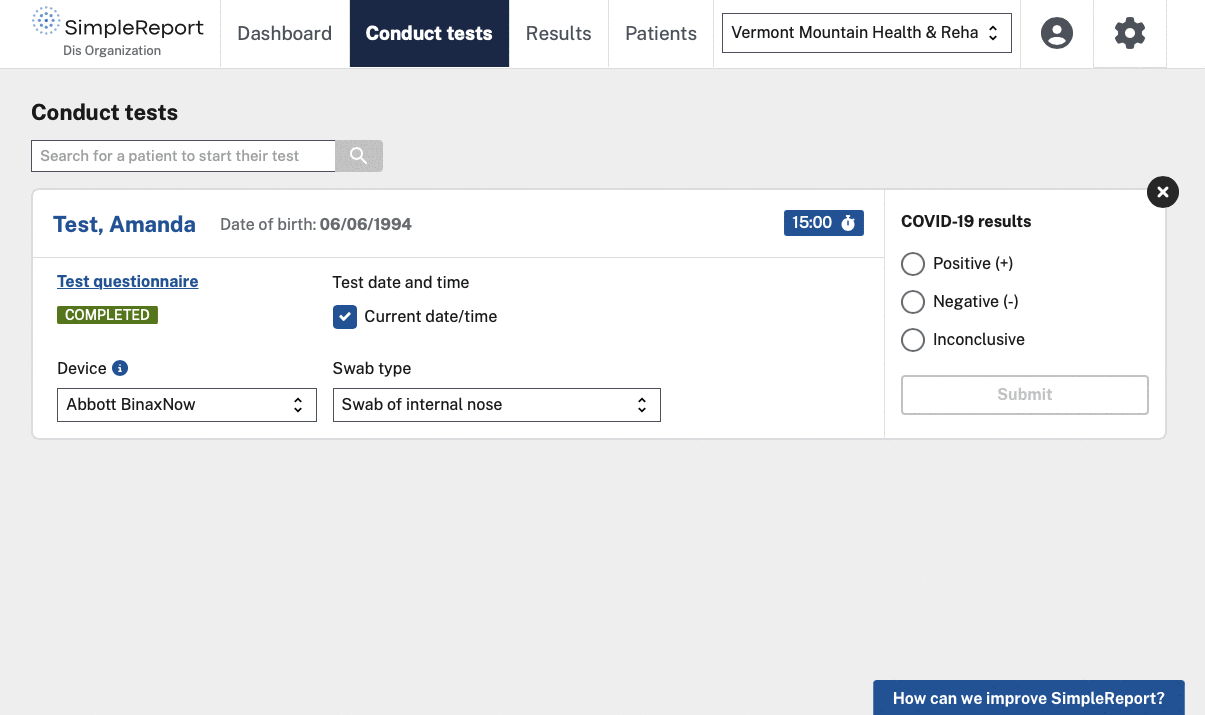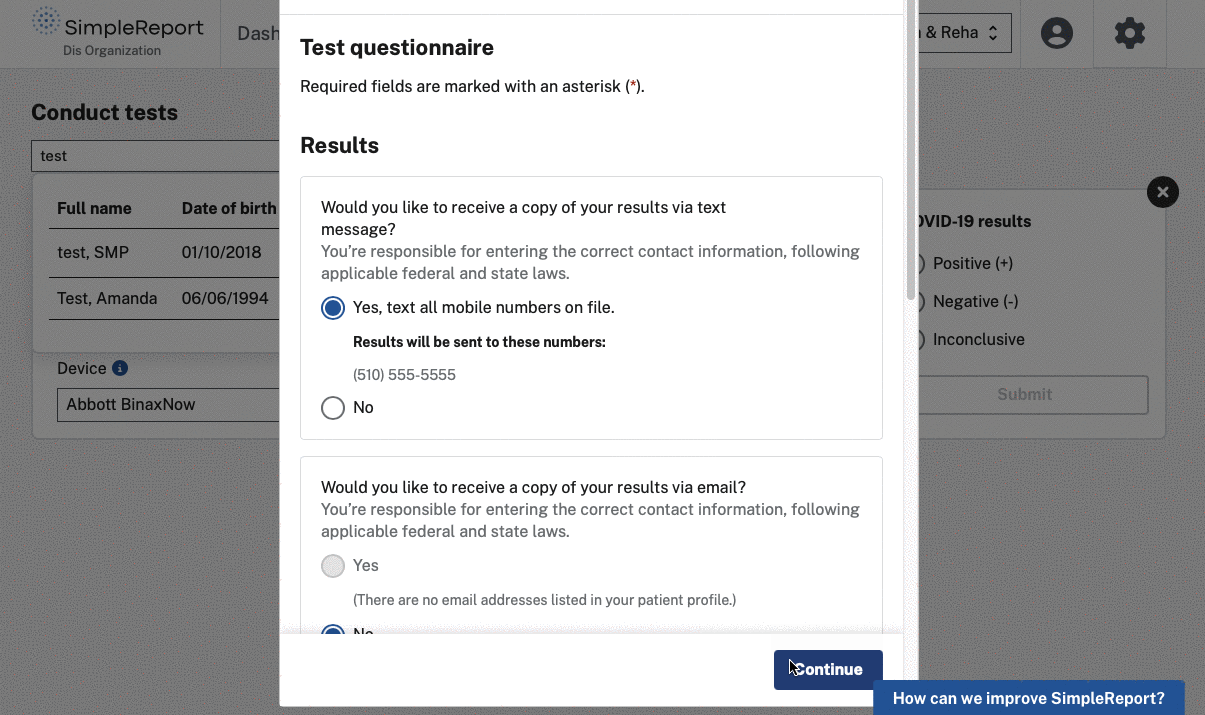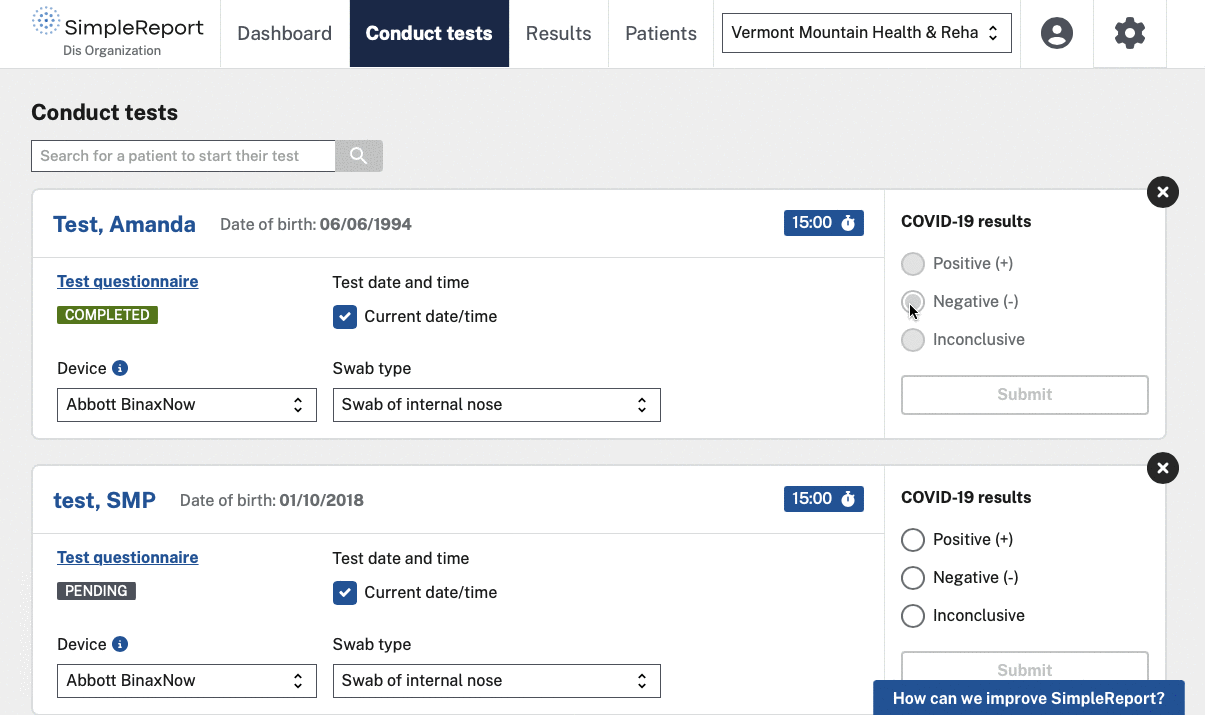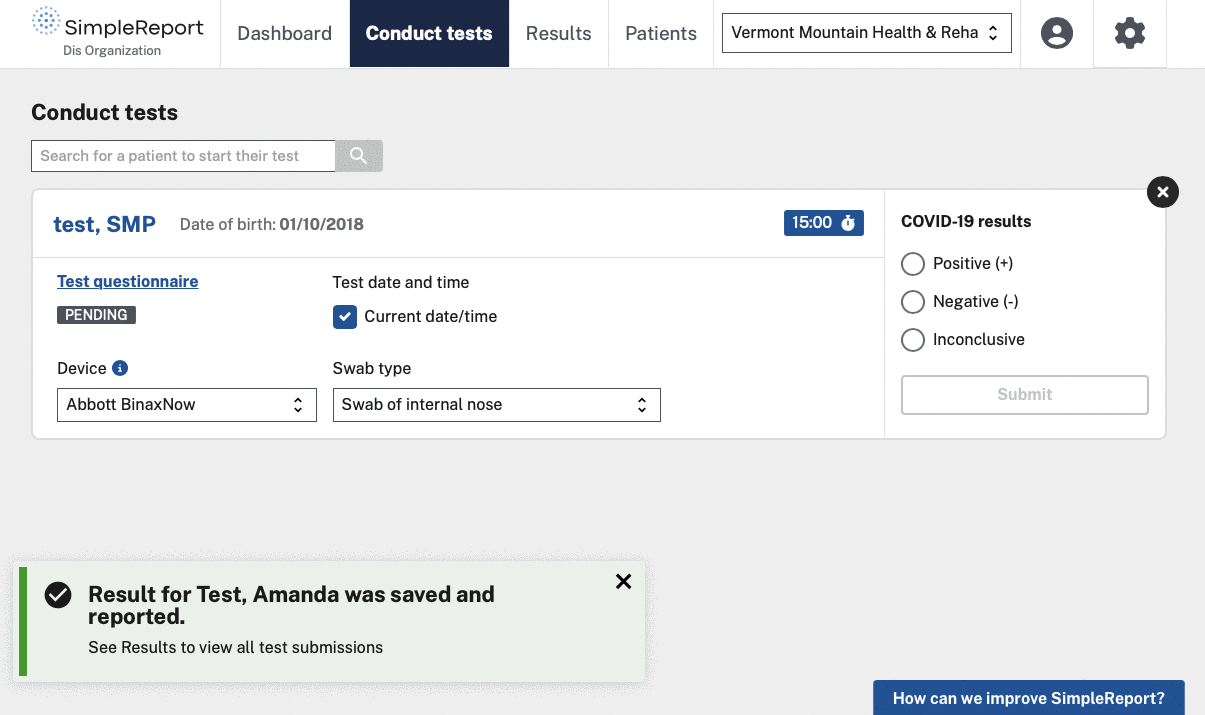
The challenge
To respond to disease outbreaks quickly, public health departments need complete, reliable, and actionable disease incidence data. For example, during the COVID-19 pandemic, the test positivity rate informed policy interventions, such as mask guidance, stay-at-home orders, and limits on indoor activities. Simply put: Better data enables public health departments to make informed decisions quicker, which helps all of us.
At the height of the COVID-19 pandemic, testing expanded outside of traditional health settings to facilities such as K-12 schools and retirement communities. However, collecting and sending accurate testing data in these settings was difficult — if it happened at all. Non-traditional point-of-care (POC) testing facilities typically lacked IT infrastructure to send data to public health departments. Many recorded test results on paper then faxed them to the appropriate department, a time-consuming process that was usually outside a test administrator’s primary job. Patients were also less likely to provide demographic information in these unconventional settings, leading to incomplete and lower-quality data for health departments.
Adding to the complexity were health departments’ differing requirements. As one interviewee who reports to multiple jurisdictions noted:
It’s a challenge to do the test, put it on paper, then go back and enter it in both formats — for the county and state.
Even when facilities did have tools to track patients and results, many lacked the ability to automatically report to their jurisdictions in the different formats that they required.
On the receiving end, public health departments had to process manual reports sent from a variety of locations, which took time and resulted in errors. This manual and cumbersome process made it difficult to act fast and stay informed.
The solution
To strengthen the infrastructure of COVID-19 testing and reporting, the Centers for Disease Control and Prevention (CDC) and the U.S. Digital Service (USDS) launched a multi-year collaboration called the Pandemic-Ready Interoperability Modernization Effort (PRIME). Skylight has supported this work by helping to launch and grow SimpleReport, a free tool that makes it easy to record results for rapid disease testing and quickly report data to public health departments.
SimpleReport is designed to be used as both a workflow and reporting tool for non-traditional and under-resourced test sites, regardless of whether or not they have a digital connection to a public health department’s disease surveillance system. SimpleReport results are automatically sent to public health departments via a single point of connection called ReportStream, which is also part of PRIME. As one interviewee described the reporting process:
Going from what we were doing to this is awesome. It’s very easy to use.
SimpleReport sends test results to public health departments in the format and at the cadence that they request. SimpleReport also provides structured data to health departments, so they don’t have to spend time on additional clean-up.
After the Skylight team fully assumed SimpleReport management from our USDS partners, we’ve continued making the tool as easy to use as possible while expanding its functionality. Our team focused on continually improving and growing SimpleReport through:
- Enabling scaling by redesigning the account creation, login, and password reset experiences, which sped up onboarding while maintaining security
- Focusing on health equity by providing patient-facing Spanish content and collecting better demographic data with tools for patients to self-register
- Proving the ability to support other diseases by adding test result recording for multiplex devices, which test for both COVID-19 and flu
- Growing SimpleReport’s reporting capabilities by adding and iterating on a feature that allows users to upload test results in bulk using a CSV file
- Easing reporting that facilities need to do beyond public health, including adding one-click results download and an analytics dashboard
- Maintaining high performance by implementing automated service monitoring; working closely with the CDC support team to resolve end-user issues; and providing 24/7 on-call engineering support
- Using continuous integration and continuous deployment (CI/CD) practices for secure and fast development — for example, when code is pushed to production, it’s merged in 20 minutes
- Ensuring SimpleReport meets high accessibility standards by integrating reviews in design and engineering workflow
As we continue to support ongoing COVID-19 reporting, we’re also focusing on pandemic readiness and filling in gaps in other public health reporting. To further this goal, the team has expanded SimpleReport for use with additional infectious diseases beyond COVID-19. To date, we have:
- Worked with California’s Department of Public Health to support the reporting of flu and respiratory syncytial virus (RSV) results from facilities who didn’t have a way to report results electronically
- Established a pilot with the Los Angeles County Department of Public Health to provide a reporting solution for organizations conducting HIV and STI testing
- Created and circulated a communication plan to current and new SimpleReport users to explain and publicize the new functionality for additional diseases and provide an overview of how to report these results
In addition to reducing the burden for non-traditional and under-resourced facilities who test for conditions besides COVID-19, this work also ensures that SimpleReport has the flexibility necessary to be quickly deployed for the next pandemic. By building out and linking public health infrastructure where these connections didn’t exist before, SimpleReport, along with the other PRIME projects, is fundamentally changing how disease incidence reporting is happening in the U.S. These tools are laying the structural groundwork for efficient and effective responses to future health crises.
The results
- Over 8.2M tests have been performed since launch (from Jan 2021 to April 2024)
- Over 17,300 facilities are reporting tests through SimpleReport
- The tool has been used to test patients from all 50 states, as well as other U.S. territories and Canadian provinces
- More than 2.3M total text messages sent notifying patients of their test result
- SimpleReport was used as an example of a Data Modernization Initiative (DMI) success story in a presentation to CDC Director Dr. Walensky for its “game-changing” work in Alaska
- Following a successful pilot with California, support for flu reporting launched in July 2023 and RSV reporting went live in January 2024
- More than 1,150 facilities in five states have joined the flu and RSV pilot
- SimpleReport users have reported over 143,000 flu test results and over 4,200 RSV test results
- Support for HIV reporting is scheduled to go live in April 2024 with the Los Angeles Community Health Project
- Given the success of the tool, the CDC is exploring other use cases where SimpleReport can be used to increase reporting from the field